Aluminum modular pharmaceutical clean room
-
Supplier: Wuxi Yijing Purification Equipment Co., Ltd. -
Region: Jiangsu, China -
Contact: Ms Maylynn Huang -
Price: $200.00 /square meter -
Min. Order: 1 square meter
| Warranty: | 1 Year; | Keywords: | cleanliness; |
| Port: | Shanghai; | Packaging Detail: | Export Wooden Packing; |
| Floor: | Epoxy Floor/pvv Floor; | Door Material: | Aluminum/Stainless steel; |
| Dimension of shower area: | customized; | Clean class: | Federal Standard 209E; |
| Door Lock: | LCJ Electronic Lock; | Payment Terms: | L/C,T/T,Western Union; |
| material: | sandwich board; | Application: | pharmaceutical, medical, food, electronic; |
| Brand Name: | Yijing; | After-sales Service Provided: | Overseas service center available; |
| Function: | Sterilization; | After-sales Service Provided: | Online support; |
| Place of Origin: | Jiangsu China; | Applicable Industries: | Building Material Shops,Manufacturing Plant; |
| Supply Ability: | 1000 Set/Sets per Month; |
What is a GMP Cleanroom?
GMP stands for "Good Manufacturing Practice", which is the globally accepted standard of regulations for drug, medical device, and certain food manufacturing set forth by the American FDA (Food & Drug Administration). Therefore, cleanrooms that adhere to GMP standards are a must for drug and medical device manufactureres.
The GMP standards apply to the areas of starting materials, employee management, facilities and equipment, manufacturing processes, packaging, product quality, and complaint handling. The aim of these standards is to reduce contamination and increase quality of products critical to the health and safety of everyone. In order to accomplish these benchmarks, companies that produce these critical products must employ acceptable equpiment, a consistent manufacturing process, strict quality management, and constant process improvement. Our GMP Cleanrooms can help your enterprise meet the GMP standards by installing a state-of-the-art cleanroom solution that, when paired with an adequate management system, will minimize potential contaminants in your products.

GMP Cleanroom Construction Materials
GMP requires a cleanroom or clean area for pharmaceutical manufacturing. Within the GMP there are further standards for the materials used in the construction of a cleanroom or area compliant with GMP. All materials must be able to withstand frequent cleaning and disinfection. Additionally, materials must be free of cracks, smooth and level, and able to interlock seamlessly. Particulate materials may not be used due to the risk of contamination. Aside from GMP compliance, other factors such as durability, ease of installation, and cost come into consideration when designing a cleanroom. At E-Clean we will guide you through the design process step by step to ensure that both GMP standards, your needs, and your budget are all satisfied.
GMP Cleanroom Floors
(1) Cleanroom floors are required to be smooth and level, scratch resistant, impact resistant, easy to clean, high integrity, and free of cracks. In addition, GMP cleanroom floors must be anti-static and possess sufficient weight bearing capacity.
(2) Cleanroom floors must be moisture resistant. To achieve this, install a buffer layer below the concrete foundation layer and use an elevated floor.
(3) Approved floor surface coatings: a) Inelastic Surface - Terrazzo / b) Painted Surface (film) - acrylic, epoxy, polyurethane; self-leveling - self-leveling epoxy resin / c) Elastic Facing Material - PVC Plastic Flooring
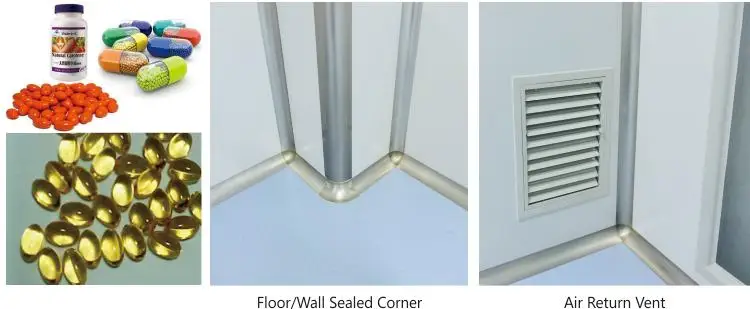
GMP Cleanroom Walls
Cleanroom walls and ceilings should be bright and clean, fade resistant, smooth and even, and easy to clean. Common materials for cleanroom walls include stone, Choi steel, and Terrazzo, which are often coated in a special paint. For especially damp production environments that are difficult to keep clean, Vitrolite (porcelain) walls may be used. However, in this case it is crucial that the material is applied evenly and sealed with no cracks to minimize the risk of contamination. We recommend Choi steel and/or paint coating for best results.
GMP Cleanroom Doors and Windows
Doors used in cleanrooms must be smooth and even, easy to clean, of simple design (no door sill), and must open in the direction or the cleanest area that they connect. Cleanroom doors must be sealed when not in use to preserve the positive pressure environment. In order to avoid mold and deformation, wooden doors should never be used in clean areas or cleanrooms.
Windows in cleanrooms should be smooth and even, of simple design, resistant to dust collection, and installed flush with interior walls (no windowsill). Cleanroom windows should not be made of wood. Windows installed between clean areas and non-clean areas should consist of at least two layers, with at least one of those layers being a non-moveable pane.

GMP Cleanroom Requirements for Health Products
(1) An appropriate air quality and temperature control system must be installed according to the type of health product being manufactured and the proximate environment.
(2) The manufacture and packaging of pills, tablets, caplets, soft capsules, powders, medicinal teas, pastes and related products should abide by the guidelines set forth in GMP for D level cleanrooms. The manufacturer may further optimize the manufacturing and packaging environment and facilities depending on the exact nature of the product.

GMP Cleanroom Technical Specifications
GMP classifies cleanrooms/clean areas into four levels: A, B, C, and D. These levels relate to how effective the cleanroom/clean area prevents contamination of the manufacturing and/or processing environment by dust particles and microorganisms. The four levels also have set ranges for temperature control and air pressure control, as well as lighting and noise levels.
Level A: High-priority or high-risk areas such as filling areas, bottling areas and packaging areas. Laminar flow hoods are often used in A-level areas in order to avoid turbulent air flow, which increases risk of contamination. Level A areas must maintain uniform air flow at a speed of between 0.36 - 0.54 m/s.
Level B: Level B cleanrooms/clean areas are common in sterile configuration areas and filling areas, as well as in background areas adjacent to A-level areas.
Levels C & D: Levels C and D are common in lower risk areas throughout the manufacturing and packaging process that do not have cleanliness standards as stringent as those in levels A and B.

GMP Cleanroom Procedures for Entry/Exit
Anyone who enters a Level D cleanroom must change their shoes, put on cleanroom apparel, and put on a facial mask. For levels A-C, the following procedure must be followed:
Take off shoes, take off clothes, shower, put on cleanroom apparel, wash and disinfect hands, enter cleanroom through air shower.

What can an E-Clean GMP Cleanroom do for your Company?
(1) Our many years of experience providing cleanroom solutions for the pharmaceutical industry have provided us deep insight into meeting our clients' needs and budgets, while also designing and constructing facilities that meet the highest international standards.
(2) We prioritize energy savings in order to reduce your future operating costs.
(3) We can design cleanroom solutions to meet any international standard, including ISO14644, FS209E, IEST and EN1822.
(4) We don't just do cleanrooms. We take into account your entire operation--from the flow of people to facility logistics the ventilation system--in order to ensure that your enterprise executes at maximum efficiency.
(5) We can design, install and test every aspect of your purification system. We also educate you on how to maintain your cleanrooms and equpiment to ensure future compliance with relevant standards.

E-Clean Certificate

Selected E-Clean Cleanroom Projects
Zhanjiang, China - De Tang Pharmaceutical, 10,000-level (ISO 7) Production Facility
Guilin, China - San Jin Pharmaceutical, 300,000-level Tablet Production Facility
Inner Mongolia, China - Li Kang Antibiotics, 100,000-level (ISO 7) Production Facility
Kunming, China - Qun Xin Pharmaceutical, 300,000-level Caplet and Pill Production Facility
Dandong, China - Tong Yuan Pharmaceutical, 300,000-level Caplet Production Facility
Jiangxi, China - Jiu Feng Tong Fang Pharmaceutical, 300,000-level Production Facility
Zhangjiagang, China - Gang Hao Bo Chemical (Chinese-American JV), ISO 7 Production Facility
Wenzhou, China - An Ji Bottled Water, 1,000-level (ISO 6) Filling Facility
Shanghai, China - #411 Naval Hospital, Chinese Medicine Prep Workshop
Luoyang, China - Luoyang City Hospital, Infusion Prep Room
Luoyang, China - Luoyang No. 4 People's Hospital, Prep Room
Taihe, China - Taihe People's Hospital, Infusion Prep Workshop
Wendeng, China - Preventative Medicine Hospital, Capsule/Tablet Prep Room
Jiangsu, China - San Tai Beer Co., 1,000-level (ISO 6) Filling Facility
Ningbo, China - Tian Ding Biological Technologies, 100,000-level (ISO 8) Health Product Production Facility
Jiaxing, China - Mei Dan Food Co., 300,000-level Sterile Filling Facility
Zhejiang, China - Zheng Wei Food Co., 10,000-level (ISO 7) Flavoring Facility
Yanzhou, China - Shandong Xin Lu Biological Technologies, Pharmaceutical Production Facility
Laiyang, China - Bao Li Yong Medical Products Co., 100,000-level (ISO 8) Production Facility
Jiangsu, China - Ao Qi Hai Yang Biological Processes Co., 10,000-level (ISO 7) Caplet Production Facility
India - Bao Li Yang Medical Products, 100,000-level Production Facility
Wenzhou, China - Ben Cao Yuan Biological Technologies, Makeup Production Facility
Changxing, China - Dong Sheng Biological Technologies, 100,000-level (ISO 8) Medical Product Production Facility
Jiangyin, China - Jin Tai Ke Biological Technologies, 10,000-level (ISO 7) Dental Product Production Facility
Wuxi, China - Xin De Rui Food Technologies, 100,000-level (ISO 8) Cleanroom
Nanjing, China - Rui Ji Ke Biological Technologies, 10,000-level (ISO 7) Sterile Laboratory
Ningbo, China - Xiu Lin Biological Technologies (Korean), 1,000-level (ISO 6) Laboratory
Kazakhstan - 100,000-level (ISO 8) Pharmaceutical Production Facility
E-Clean with Clients



E-Clean with other products

About Us
With more than a decade of experience in the cleanroom and industrial purification equipment sectors, E-Clean (Wuxi Yijing Purification Equipment Co, Ltd.) is an industry leading company located west of Shanghai in the city of Wuxi, Jiangsu, China. E-Clean offers certified cleanroom design, manufacture, and installation services compliant with ISO 14644 and FS209E standards, as well as top of the line industrial purification equipment such as air showers and clean workbenches. Our professional team of engineers will customize your clean room project according to your specifications, and E-Clean can even provide on-site installation and quality testing services. Whether your company is looking to upgrade its purification equipment or construct new cleanroom facilities, E-Clean can help you execute cleanly from start to finish. Contact us today for a free consultation and estimate of your company's next cleanroom project.


-
Laminar flow hood laminar flow hood anti-static laminar flow hood 4*2' Ffu Hepa pre-filter Ffu fan filter unit Hepa

-
BIOBASE China Workstation Pathology Explosion-proof and Chemical-proof Exhaust Workstation

-
ISO CE 100 Certified Laminar Flow Cabinet, ISO 5 Laminar Flow Hood Clean Bench/Automation Laminar Flow Hood

-
FFU Unit Fan Filter Unit with FFU HEPA Filter

-
Laminar Flow Hood FFU Air Purifier Laminar Flow Hood Mycology Cleanroom

-
Class 100 Laboratory Horizontal Laminar Flow Hood with Hepa Filter Laminar Flow Cabinet Clean Bench

-
Pass Box Clean Room Window Clean Room Clean Room Pharmaceutical Pass Box

-
Suction chamber absorbs overspray

-
ISO 5 Laminar Flow Cleanroom Air Filter Aspirator

-
OEM High Cleanroom Cleanroom Mini Modular Booth Cleanroom

Other Products
-
 $1000.00 / unit
$1000.00 / unit -
 $3120.59 / set
$3120.59 / set -
 $1000.00 / set
$1000.00 / set














